Initial Submission
Investigators are responsible for ensuring IRB approval is obtained.
Timing: Investigators must submit and receive approval prior to beginning any research activity.
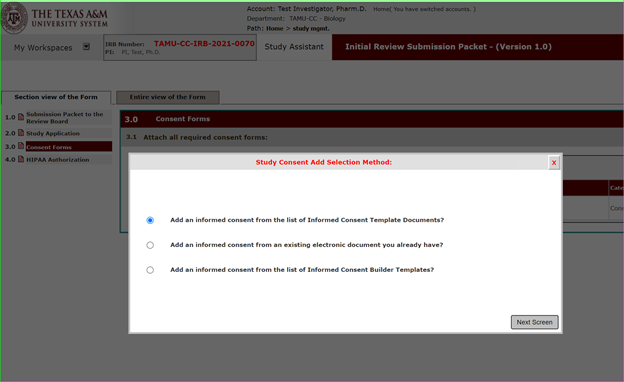
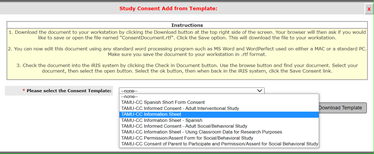
You cannot begin recruitment or request the written informed consent of any subject to participate before obtaining IRB approval. 21 CFR 812.110; ICH 3.3.6.
How do I start a new submission in iRIS?
- Login into iRIS.
- On the study assistant dashboard, select “Create a New Study” to open the online application.
- Click to learn more about submitting an initial submission